Systems Biology of Proteostasis and the Unfolded Protein Response
 A schematic model of the unfolded protein response. Source: W Stroberg et al. (2018) Molecular Biology of the Cell 29, 2969-3062.
A schematic model of the unfolded protein response. Source: W Stroberg et al. (2018) Molecular Biology of the Cell 29, 2969-3062.
Approximately 1/3 of proteins in eukaryotic cells originate in the endoplasmic reticulum (ER). The proper folding of proteins within the ER requires the coordination of chaperones and foldases to guide nascent proteins to the correct conformation. Should the folding environment within the ER be perturbed, the cell must respond to restore proteostasis, or else risk accumulation of misfolded proteins. The inability to do so has been implicated in numerous protein folding diseases such as Alzheimer’s and Parkinson’s disease, as well as diabetes and cancer.
The main regulatory mechanism by which cells control the ER environment is the unfolded protein response (UPR). The UPR is a coordinated set of regulatory responses that originate from three signaling proteins in the ER membrane, and seeks to mitigate unfolded protein “stress” by increasing the folding capacity of the ER and decreasing translation. Should these interventions prove insufficient, the UPR initiates apoptosis.
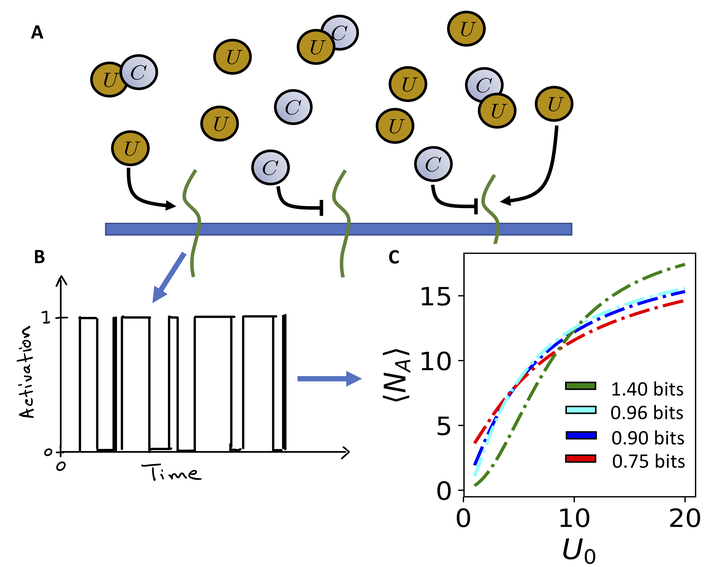
I am interested in understanding how cells determine how stressed they are and how they coordinate a diverse array of responses to mitigate that stress. The dynamic nature of the ER chemical environment, shape and membrane composition makes stress signaling particularly complex. The response is equally complex, altering the folding propensity, quality control, and export from the ER. To develop a theoretical understanding of the protein secretory process across multiple scales, I employ techniques from control theory, signal processing and information theory, chemical kinetics and molecular simulation.